The Fundação D. Anna de Sommer Champalimaud e Dr. Carlos Montez Champalimaud (Champalimaud Foundation), was created by António de Sommer Champalimaud in his will, being a legal person of private law and public utility, governed by the statutes published in Diário da Republic of January 21, 2005 and by Portuguese Law.
The Champalimaud Foundation conducts cutting-edge research and prioritises the stimulation of discoveries that benefit people, as well as supporting new standards of knowledge.
It is at the Champalimaud Centre, in Lisbon, that it develops its activity in the areas of neuroscience and cancer, through research programmes and the provision of excellent clinical services, as well as an external programme to fight blindness.
To pursue its objectives of achieving significant scientific advances, the Champalimaud Foundation adopts a translational methodology, which establishes a direct and interdependent link between basic research and clinical activity. This is one of its fundamental operating principles and on which its differentiation is based.
Ultimately, the aim is to promote the health and well-being of humanity, seeking to actively intervene in the search for solutions that alleviate the burden that ill-health has on societies and on the individual.
The Champalimaud Foundation (CF) is a private non-profit organisation, founded in 2005, dedicated to the development of advanced biomedical research programmes and the interdisciplinary provision of clinical care. Through its activities, the Foundation intends to be a world leader in scientific and technological innovation with the ultimate goal of preventing, diagnosing and treating disease, guided by a posture of constant challenge and contributing to a society that is more aware of the health problems that affect humanity.
Completed in 2010, the Champalimaud Centre for the Unknown (CCU) is a state-of-the-art centre dedicated to basic and translational research as well as the diagnosis and treatment of diseases ranging from neurological disorders to cancer. Comprising the Champalimaud Clinical Centre (CCC) and Champalimaud Research (CR) with their three research programmes - the Champalimaud Neuroscience Programme (CNP), the Physiology and Cancer Programme (PCP) and the Clinical Experimental Research Program (CERP) ) -, the Champalimaud Foundation offers a unique environment for conducting cutting-edge research, aimed at revealing new knowledge and developing scientific solutions to medical problems.
The multidisciplinary teams of the Champalimaud Clinical Centre (CCC) focus their activity on the maintenance of highly personalised medicine, centred on the patient and of a multidisciplinary nature, following standards of excellence and always within the perspective of scientific research and rapid transfer to clinical progress and scientific innovations. It is also a constant concern to respect deontological and ethical principles of good medical practices and defence of the well-being and quality of life of those who seek our help. With the progressive increase in clinical activity, the work of research groups is often consolidated through strong interaction with clinical groups in related areas, namely in the domain of ontogeny and biopathology of neoplastic transformation and metastasis, of cancer immunophysiology and the therapeutic response to different modalities of immunotherapy.
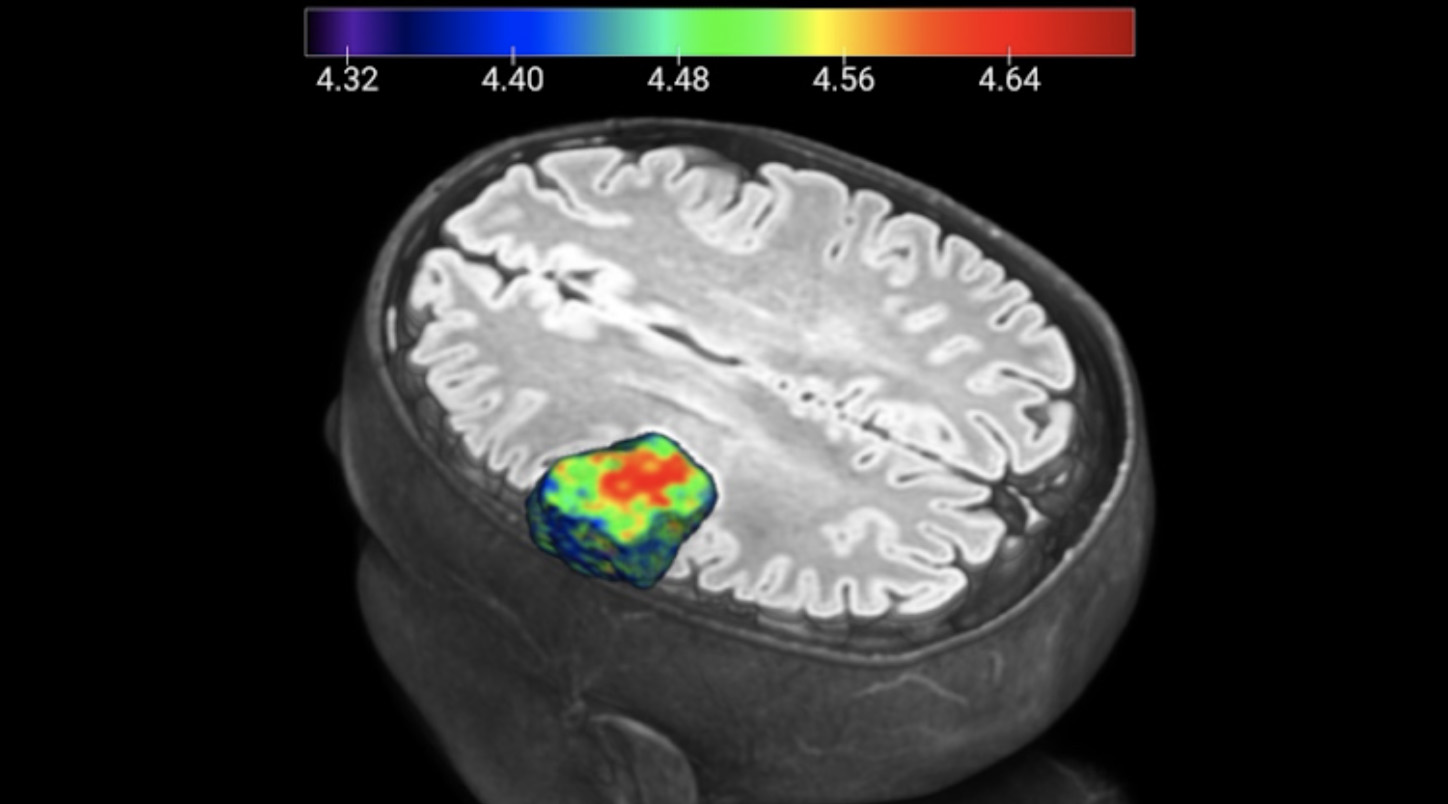
The CCC has more than 600 professionals, including 138 physicians, organised into seven multidisciplinary pathology units, whose main objective is to make all the knowledge and techniques available for the practice of personalised and results-oriented medicine, aimed at different types of tumours, including breast, digestive, gynaecological, haemato-oncological, lung, genitourinary and dermatological. Risk assessment, early diagnosis of disease and treatment personalisation are the main concerns of all multidisciplinary units, in order to achieve better levels of effectiveness in controlling the disease, survival and quality of life for the patient. Whenever possible, CCC's clinical staff offers patients the opportunity to participate in clinical trials or experimental research programmes aimed at creating innovative diagnostic and treatment solutions, through the CCC's Clinical Research Unit. Clinical studies performed are predominantly in the area of cancer (including breast cancer, gynaecological, lung, prostate and kidney, digestive and haemato-oncology) and neuropsychiatry. In these areas, studies are also conducted focusing on radiotherapy, radiology and nuclear medicine. With the increase in clinical activity and research projects dedicated to pancreatic cancer, driven by the opening of the Botton-Champalimaud Pancreatic Cancer Centre, a significant increase in the number of clinical studies focusing on understanding and treatment of this type of cancer is expected in the near future.
The Clinical Trials Unit (CTU) team has ten coordinators, with whom it was possible, in 2020, to maintain several active clinical trials and to collaborate in the conduct of observational studies already under development in previous years, as well as others that began in 2020. 37 clinical pharmacological trials were carried out in the different areas of oncological pathology, alongside 43 observational clinical studies. The total number of patients recruited for the ongoing clinical trials was 348, of which 24 are in active treatment. Observational studies involved a total of 1,865 patients
The CCC also has an International Patient Office, a structure created in 2016 to support overseas patients who use the Champalimaud Foundation, having so far accompanied more than 400 patients from 38 countries.