Hospital da Luz Lisboa

Hospital da Luz Lisboa
Luz Saúde was established in 2000 and it is one of the largest health care groups in the portuguese market. The Group provides its services through 28 units and Hospital da Luz Lisboa is the biggest hospital, with 400 beds.
The Hospital da Luz Lisboa is part of an integrated medical complex consisting of an acute care hospital and a residential hospital.
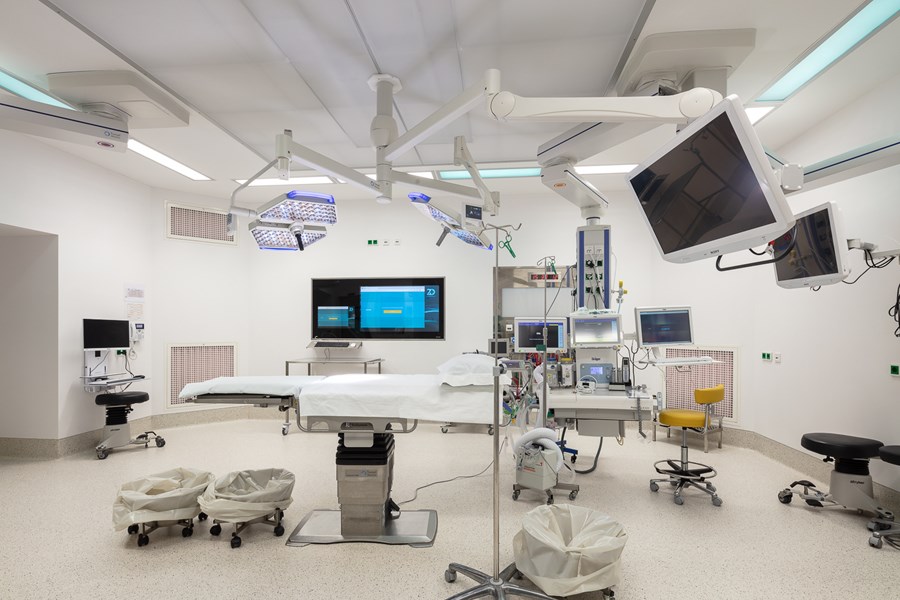
The quality and experience of the clinical staff, with a medical team with approximately 880 resident and collaborators physicians, the technological innovation of medical equipment and information systems make the Hospital da Luz Lisboa a model of medicine of excellence and innovation on a European level. Also, its unique architecture combines safety with comfort and privacy of patients.
Hospital da Luz Lisboa offers all medical and surgical specialties, with emphasis in areas organized in multidisciplinary centers of excellence, contributing to a comprehensive and integrated approach to patient. Among others:
- Oncology
- Cardiovascular diseases
- Diabetes
- Obesity
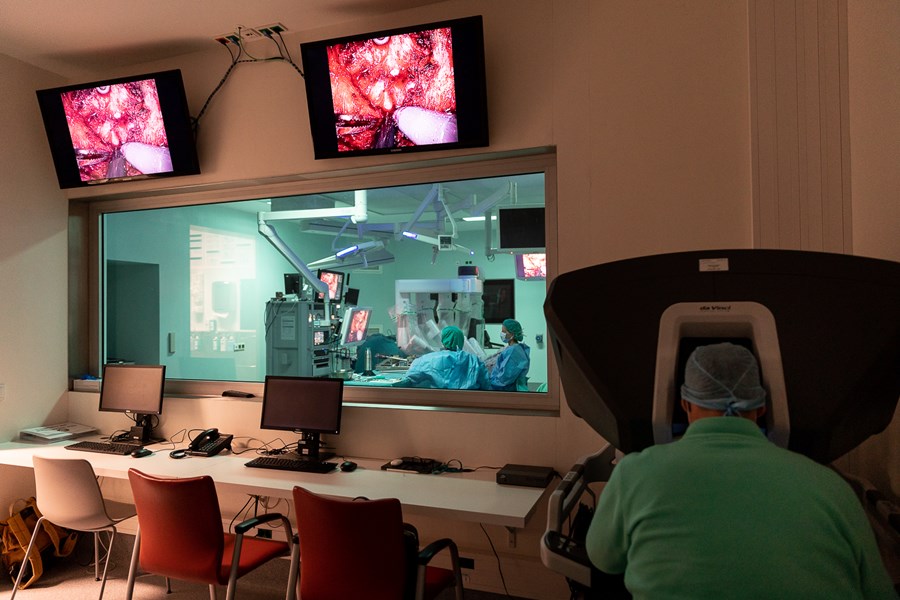
- Robotic surgery
- Headaches
It has been accredited since 2018 by JCI, with a global compliance exceeding 98% in relation to the highest hospital standards defined by this international organization.
The Hospital da Luz Learning Health is dedicated to the advanced training of professionals, translational research, innovation and medical simulation for the Luz Saúde group.
In the area of research, the Hospital of Luz Learning Health:
- Develops, promotes and supports the research developed in the Luz Saúde group;
- It stimulates the development of new methodologies that add value to the care provided to our clients.
We have a team of 11 employees, made up of clinical trial coordinators, project managers, contract managers and research grant managers.
In 2019, Luz Saúde opened an internal competition to fund projects from the investigators' initiative, a strategic investment of the group in clinical research. These grants are intended to contribute to the promotion, structuring and professionalization of research in the group.
Two important and simplifying tools created by Learning Health stand out:
- Internal submission of clinical trials 100% online
- Electronic trial monitoring tool, which allows monitors a direct, read-only access to the clinical file exclusively for participants in that trial.
Video
General Indicators
-
46Active Clinical Trials
-
42Active Non-Interventional Studies
-
576No. of publications in indexed journals - last 5 full years
-
200No. of health professionals with specific training in GCP (Good Clinical Practices)
Global Capacity Indicators
-
400No. of available beds in the institution
-
880No. of Physicians in the Institution
-
387No. of Nurses in the Institution
-
14No. of Pharmacists in the Institution
HR Indicators Allocated to Research
-
40Researchers
-
5Managers, Administrative, Legal and Financial
-
4Study coordinators
-
10Nurses
-
5Pharmacists
Efficiency Indicators
-
121Total number of participants recruited for clinical trials and interventional clinical studies of medical devices
-
70 daysAverage time between submission and approval of the Financial Contract in clinical trials and interventional clinical studies of medical devices
General Indicators
-
44Active Clinical Trials
-
24Active Non-Interventional Studies
-
467No. of publications in indexed journals - last 5 full years
-
150No. of health professionals with specific training in GCP (Good Clinical Practices)
Global Capacity Indicators
-
400No. of available beds in the institution
-
880No. of Physicians in the Institution
-
387No. of Nurses in the Institution
-
14No. of Pharmacists in the Institution
HR Indicators Allocated to Research
-
40Researchers
-
5Managers, Administrative, Legal and Financial
-
4Study coordinators
-
10Nurses
-
5Pharmacists
Efficiency Indicators
-
121Total number of participants recruited for clinical trials and interventional clinical studies of medical devices
-
70 daysAverage time between submission and approval of the Financial Contract in clinical trials and interventional clinical studies of medical devices
General Indicators
-
48Active Clinical Trials
-
52Active Non-Interventional Studies
-
680No. of publications in indexed journals - last 5 full years
-
200No. of health professionals with specific training in GCP (Good Clinical Practices)
HR Indicators Allocated to Research
-
70Researchers
-
8Managers, Administrative, Legal and Financial
-
5Study coordinators
-
18Nurses
-
11Pharmacists
Efficiency Indicators
-
87Total number of participants recruited for clinical trials and interventional clinical studies of medical devices
-
70 daysAverage time between submission and approval of the Financial Contract in clinical trials and interventional clinical studies of medical devices
Clinical Research Areas of interest
Clinical Research Areas of interest
- Oncology
- Cardiology
- Neurology
- Gastroenterology
- Immunoallergy
- Virology
- Imaging
- Hematology
- Ophthalmology
- Endocrinology
Certifications
Certifications
- SIFIDE suitability for Research and Innovation by the National Innovation Agency (Learning Health)
- Joint Commission International Accreditation 2021
- ISO 9001 for Pathology Lab, Nuclear Medicine and Imaging
- European Association of Nuclear Medicine Accreditation for Nuclear Medicine
- European Echocardiography Association Accreditation for Echocardiography lab
- Since 2016 Reference Center in Adult Oncology for Rectal Cancer by the DGS
Latest News
-

Luz Saúde investis the most in R&D in Health Sciences
The 2021 Enquiry on the Scientific Potential reveals the investments of hospital institutions in Research and Development. -
CAC Católica Luz: the 1st non-state clinical academic centre
The new centre, partnership between Hospital da Luz and U. Católica, views to bring together education, research and healthcare. -

Chron’s disease: Hospital da Luz promotes international clinical study
Over 20 European hospitals collaborate in this study at the initiative of Joana Torres. Patient recruitment opened this month.